
Publications
Laser diagnostics
Engine research
Flame spectroscopy
Astronomy & Atmospheric optics
TI-58/59
Contact
Home

True colour optical emission spectrum of an oxyacetylene flame
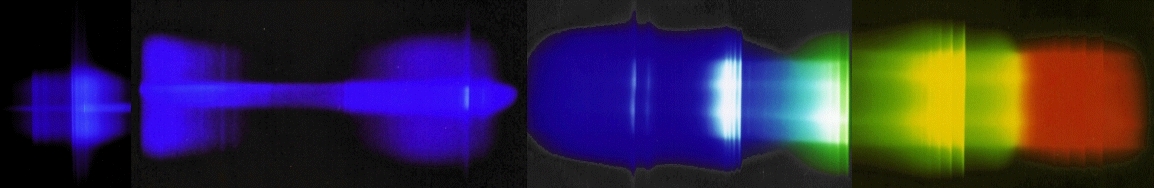
Combination of 4 photographs (on colour slide film) taken through a
spectrograph. The exit slit is removed and the camera lens as well, so
the light falls directly onto the photographic film. The photographs
reveal in true colours the emission spectrum of the oxyacetylene
(C2H2/O2) flame from the UV to the
(visible) red. In contrast to the human eye, the colour slide film used
is sensitive to the ultraviolet light (which it turns into blue). All
lines visible in these photographs are identified in the graph shown
below. Please note that the OH structure around 310 nm is shown twice
(far left on a smaller vertical scale).
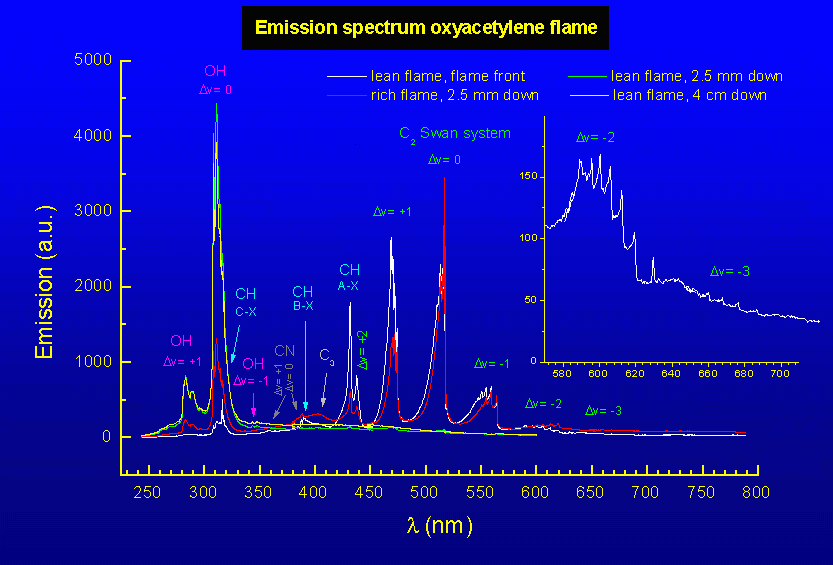
Spectrum of the natural emission of the flame front, the acetylene
feather, and the outer flame of the oxyacetylene flame. The last two are
recorded about 2.5 mm below the tip of the flame front. Since the outer
flame envelopes the flame front and acetylene feather, its spectral
features can be distinguished in their spectra as well. The position for
CN emission is indicated as well, but at the low resolution used here,
it is masked by CH emission.
Same graph in (almost) black and white (click graph to enlarge).

Short explanation of the (photographed) spectrum
The flame front of the oxyacetylene flame appears blue-green to the
naked eye, and the acetylene feather looks a little more whitish. The
emission of the flame can be dispersed to obtain a spectrum
(Newton1730), which shows that the flame emits light from 250 nm
(ultraviolet) to 800 nm (infrared), as is depicted in the figures above.
The spectrum of the oxyacetylene flame and the origin of the excited
species responsible for it are discussed in detail in the book by Gaydon
(Gaydon1974), but the most important characteristics can already be seen
in the figures and photographs above.
Emission of the flame front consists of the C2 Swan system
(between 434 and 720 nm) and the CH (A, B, C) ->X systems (main emission
around 431, 389, and 314 nm, respectively). In the acetylene feather,
emission of these two species is still the most important, but
C3 emission around 405 nm, the Comet-Head group, becomes
apparent as well. The most important radiating molecule in the outer
flame is OH. Its emission lies between 260 and 350 nm and is so strong
it causes actinic conjunctivitis ("welding blindness") if the
human eye is not protected. Due to the high temperature of the flame,
high rotational and vibrational levels of OH are well populated, which
is why emission of the oxyacetylene flame has been used to tabulate OH
transition frequencies to a large extent (Dieke1962).

![]()



![]()