Publications Laser diagnostics Engine research Flame spectroscopy Astronomy & Atmospheric optics TI-58/59 Contact Home
![]()
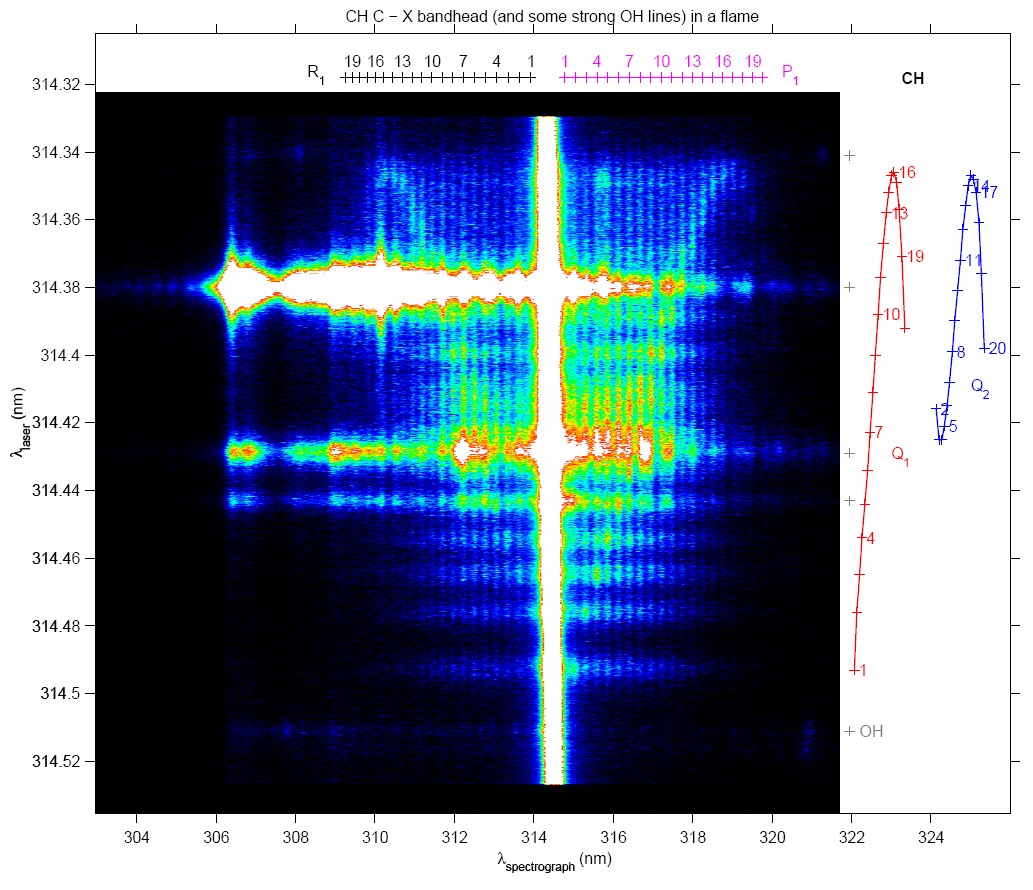
While scanning the wavelength of an ultraviolet laser over the band head of the Q branch of the C - X (0,0) transition of the CH radical (as present in a methane - air flame) and recording the laser-induced fluorescence through a spectrograph, various molecular spectroscopic processes can be observed.
The rotational quantum number N of the excited level increases from bottom to top in the image and with it the wavelengths of maximum fluorescence (horizontal scale) separate themselves further and further from the excitation wavelength. Fluorescence does not only originate from the excited level, but due to rotational energy transfer also neighbouring levels give rise to fluorescence. Above about N = 11, however, rotational energy transfer appears to be much less. The signal reflects the ground state population distribution in the CH radical, from which a flame temperature can be derived. Some transitions in the OH radical are excited as well in this wavelength range (partly overexposed here), which reveal both vibrational and rotational energy transfer to occur in OH as well (the white vertical [overexposed] stripe is due to resonant fluorescence and Rayleigh scattering of the laser light).
Using this laser excitation - detection technique, the NCN radical has recently been detected in methane - air flames at atmospheric and low pressure (see below).
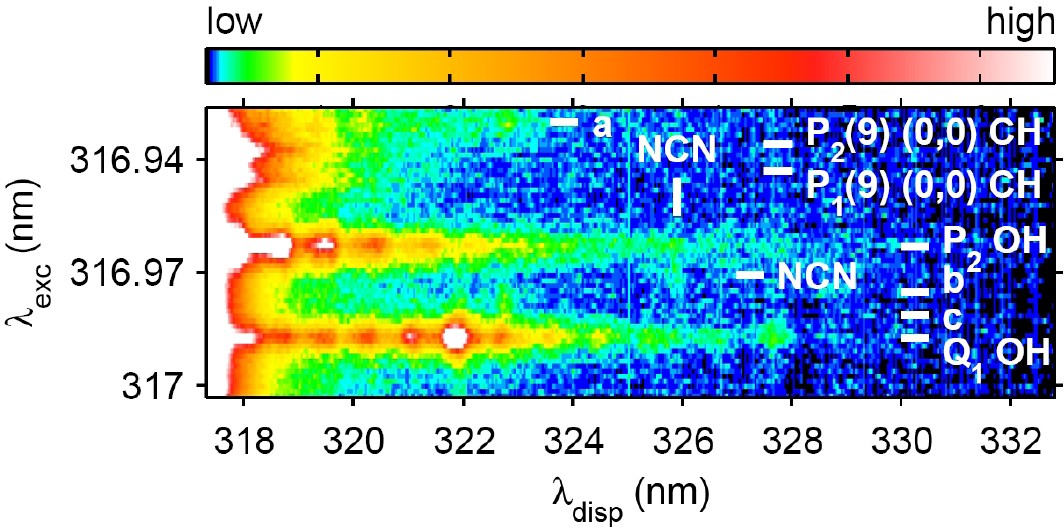
Laser excitation - dispersed fluorescence spectrum revealing NCN (cyanonitrene) signal at the flame front of a 200 hPa methane/air flame. Main LIF features originating from NCN, CH and OH are identified ('a' is due to OH and 'b/c' are due to CH).
NCN plays an important role in the formation of NO, which is emitted as a pollutant from almost all combustion processes. Comparison of our experimental results with models will hopefully allow better understanding and ultimately reduction of pollutant emissions from combustion processes.
This is the colour version of Fig. 1 in our paper Opt. Lett., 33, 2620 - 2622, (2008) (see here).
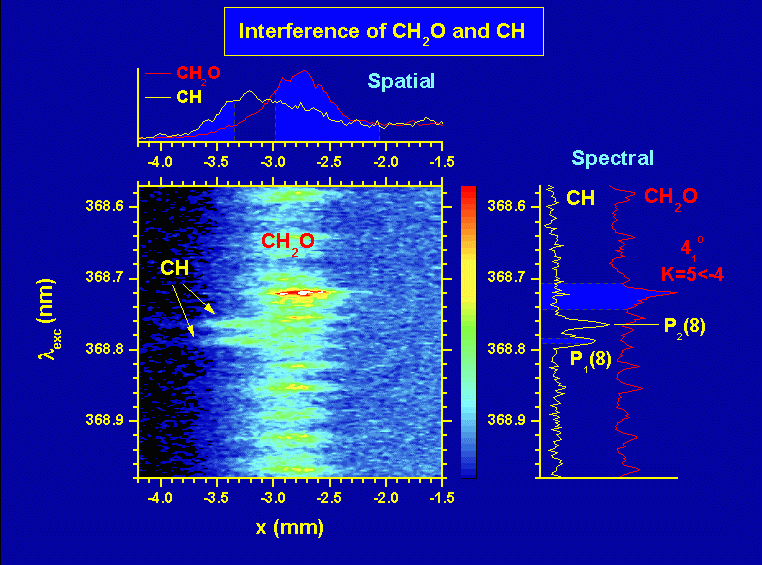
An example of near simultaneous excitation and detection, both in real space and in wavelength, of CH2O (formaldehyde) and CH (methylidyne) LIF in a Bunsen flame. The vertical axis gives the excitation wavelength and the horizontal axis gives the distance from the flame axis, so formaldehyde is present closer to the flame axis (id est where the cold fuel is) than CH.
This image is the colour version of Fig. 3 in our paper Appl. Phys. B, 75, 779 - 790, (2002) (see here).
More information and two-dimensional flame images of CH2O are given in our paper Appl. Opt., 39, 3712 - 3715, (2000) (see here) and results about quantitative LIF measurements of CH in an atmospheric pressure flame can be found in our paper Appl. Phys. B, 75, 779 - 790, (2002) (see here).
An example of LIF of the CN radical (false colours) during diamond deposition on a molybdenum substrate (bottom) in a slightly carbon-rich oxyacetylene flame (depicted here by isophotes). The nitrogen necessary for CN formation is provided by the ambient air. Nitrogen is also incorporated into the growing diamond layer. Other species studied by LIF during diamond deposition are H, NO, OH, C2 and CH.
Typical example of a flame deposited polycrystalline diamond layer (electron microscope photograph). Diamond layer morphology and other characteristics vary across the diamond layer. Relations between gas phase species distributions and properties of the flame deposited diamond layers are described my Ph.D. thesis and our papers J. Appl. Phys., 78, 2086 - 2096, (1995), Proc. Combust. Inst., 27, 2477 - 2483, (1998), J. Appl. Phys., 83, 4734 - 4745, (1998) and Diamond Relat. Mater., 7, 1118 - 1132, (1998) (see publication list).
![]()